| ||||||||
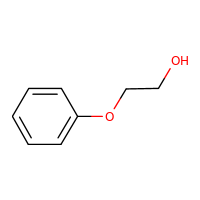
2-PHENOXYETHANOLCASRN: 122-99-6 |
 |
Human Health Effects:
Human Toxicity Excerpts:
/CASE REPORTS/ ... An 18-month-old boy who developed generalized eczema within 24 hr after administration of a DPT (diphtheria, pertussis, tetanus) vaccine. Patch tests with the individual components of the vaccine gave a positive result to PE 2% pet. ...
/CASE REPORTS/ ... A 53-year-old man with hand eczema caused by PE in an aqueous cream. Patch tests were positive to 1% PE, and the eczema improved with avoidance of the cream. ...
/CASE REPORTS/ A 24-year-old Asian woman suffered her first episode of acute urticaria 1 hr after eating papaya salad. Four days later, she again experienced generalized urticarial exanthema just after taking a shower, while applying a recently bought body lotion. She had never suffered from aquagenic or any other physical urticaria. She had no history of atopy or previous allergic reactions to food or drugs. ... Skin prick tests with common aeroallergens were all negative. The prick to prick test with fresh papaya was negative. Prick test with the body lotion gave a +++ reaction (Histamine: ++). In an open application test with the single ingredients of the body lotion and with papaya for 30 min we found a strong wheal reaction with pseudopods to PE. Tests with all the other ingredients and with papaya were negative. The prick test with Euxyl K 400 1% in pet. and with a dilution series of PE resulted in a ++ reaction to Euxyl K 400 and in a + reaction to 1% PE, +to 5% PE, and ++ to 10% PE. ... Observed an immediate reaction to PE with contact urticaria to a body lotion containing PE 1%. ...
/CASE REPORTS/ Peritonitis is the established term for infective inflammation of the peritoneum, whereas serositis generally refers to aseptic inflammation of a serous cavity, including the peritoneum. Serositis may be metabolic, viral, autoimmune, drug induced, genetic, allergic or granulomatous, or due to chemical antiseptics. In ... gynecological department, 4 patients had peritonitis and ascites after laparotomy. Based on the investigation...the solution used for peritoneal lavage (0.1% octenidine dihydrochloride and 2% phenoxyethanol) played a role in the tissue toxicity that caused chemical serositis with effusion.
/EPIDEMIOLOGY STUDIES/ Despite the decreased use of solvent-based paint (SBP) and increased use of water-based paints (WBP) with possible risk for microbial growth, few health studies are available. The aim was to study the symptoms and ocular and nasal biomarkers in house painters in relation to paint use and personal exposure to volatile organic compounds (VOC) and microbial VOC (MVOC) during indoor painting with WBP. All house painters from three major companies and unexposed controls (janitors from one company) were invited, 94% (N = 31) and 95% (N = 20) of non-asthmatics participated, respectively. Tear film break-up time (BUT), nasal patency by acoustic rhinometry, and biomarkers in nasal lavage (NAL) were measured at work, and a doctor's administered questionnaire was answered. Personal sampling (8 hr) of formaldehyde, VOC, and MVOC was performed in 17 house painters using WBP. House painters had increase in ocular symptoms, decreased BUT, and increased NAL-lysozyme, when compared to controls. Painters reporting mucosal irritation from WBP had less nasal patency and higher NAL-myeloperoxidase (NAL-MPO). A large proportion of the VOC consisted of propylenglycol, diglycol ethers, and Texanol. There was an association between 8-hr exposure to propylene glycol and NAL-eosinophilic cationic protein (NAL-ECP), 2-phenoxyethanol levels and reduced BUT, sum of aliphatic glycol ethers and increased NAL-MPO. Increased levels of 1-octen-3-ol, one MVOC, were related to reduced nasal patency and increase in NAL-MPO. House painters may have a risk for adverse physiological reactions in the ocular and nasal mucosa. A minority of painters susceptible to WBP can react with neutrophilic nasal inflammation. Different chemicals in the paint could cause either neutrophilic or eosinophilic inflammation, or reduced tear film stability. In addition, house painters are exposed to MVOC which may affect the nasal mucosa.
/ALTERNATIVE and IN VITRO TESTS/ In vaccines/biologics, preservatives are used to prevent microbial growth.The present study examined: (1) the comparative toxicities of commonly used preservatives in US licensed vaccines to human neurons; and (2) the relative toxicity index of these compounds to human neurons in comparison to bacterial cells. Using human neuroblastoma cells, the relative cytotoxicity of the levels of the compounds commonly used as preservative in US licensed vaccines was found to be phenol <2-phenoxyethanol < benzethonium chloride < Thimerosal. The observed relative toxicity indices (human neuroblastoma cells/bacterial cells) were 2-phenoxyethanol (4.6-fold) < phenol (12.2-fold) < Thimerosal (>330-fold). In addition, for the compounds tested, except for 2-phenoxyethanol, the concentrations necessary to induce significant killing of bacterial cells were significantly higher than those routinely present in US licensed vaccine/biological preparations. None of the compounds commonly used as preservatives in US licensed vaccine/biological preparations can be considered an ideal preservative, and their ability to fully comply with the requirements of the US Code of Federal Regulations (CFR) for preservatives is in doubt. Future formulations of US licensed vaccines/biologics should be produced in aseptic manufacturing plants as single dose preparations, eliminating the need for preservatives and an unnecessary risk to patients.
/ALTERNATIVE and IN VITRO TESTS/ Ethylene glycol ethers (EGEs) are a class of chemicals used extensively in the manufacture of a wide range of domestic and industrial products, which may result in human exposure and toxicity. Hematologic and reproductive toxicity of EGEs are well known whereas their action on neuronal cell viability has not been studied so far. In the present study, we investigated the effects of some EGEs on cell viability and on the hydrogen peroxide-induced damage in the human neuroblastoma (SH-SY5Y) cells. It has been found that 2-phenoxyethanol in a concentration-dependent manner (5-25 mM, 24 hr) increased the basal and H(2)O(2)-induced lactate dehydrogenase (LDH) release and 3-[4,5-dimethylthiazol-2-yl]2,5-diphenyl tetrazolium bromide (MTT) reduction. 2-Butoxyethanol given alone did not affect LDH release and MTT reduction but concentration-dependently enhanced the cytotoxic effect of H(2)O(2). 2-Isopropoxyethanol significantly and concentration-dependently (1-25 mM) increased the basal LDH release and attenuated MTT reduction, but did not potentiate the cytotoxic effect of H(2)O(2). Contrary to this, 2-methoxyethanol did not show a cytotoxic effect while 2-ethoxyethanol at high concentrations intensified the hydrogen peroxide action. This study demonstrated that among the EGEs studied, 2-phenoxyethanol showed the most consistent cytotoxic effect on neurons in in vitro conditions and enhanced the hydrogen peroxide action. 2-Isopropoxyethanol had also a potent cytotoxic effect, but it did not enhance the hydrogen peroxide action, whereas 2-butoxyethanol only potentiated cytotoxic effect of H(2)O(2). It is concluded that the results of the present study should be confirmed in in vivo conditions and that some EGEs, especially 2-phenoxyethanol, 2-butoxyethanol and 2-isopropoxyethanol, may be responsible for initiation or exacerbation of neuronal cell damage.
/ALTERNATIVE and IN VITRO TESTS/ Preservatives are added to many final products, such as detergents, cosmetics, pharmaceuticals and vaccines. We conducted an in vitro investigation of the apoptosis- and necrosis-inducing potential of brief applications (10 min) of four common preservatives: ethylene glycol monophenyl ether, 2-phenoxyethanol (EGPE), imidazolidinyl urea (IMU), a mixture of 5-chloro-2-methyl-4-isothiazolin-3-one and 2-methyl-4-isothiazolin-3-one (CMI/MI), and 1,2-pentanediol, a "preservative-non-preservative" best known as pentylene glycol. Using HL60 cells, we monitored the kinetics of cell toxicity with the MTT test and analysed extranuclear end points of apoptosis, i.e. phosphatidylserine exposure and nuclear fragmentation. Preservative treatment resulted in a dose-dependent decrease of cell viability. The mode of cell death was dose-dependent: necrosis occurred at high concentrations while apoptosis, shown by DNA laddering, DNA sub-diploid peak and caspase-3 activation, occurred at lower concentrations 0-24hr after exposure to a single dose: CMI/MI induced apoptosis at low concentrations (0.001-0.01%) and necrosis at high concentrations (0.5-0.1%); IMU and EGPE required higher concentrations to induce apoptosis (IMU 0.01-0.1% and EGPE 0.01-0.5%) or necrosis (IMU 0.5-1% and EGPE only at 1%). PG induced apoptosis only at 5%. Externalization of PS, a hallmark of apoptosis, occurred early in HL60 treated with low concentrations of CMI/MI and EGPE and was concomitant with the subdiploid peak in HL60 treated with PG. However, it did not occur in HL60 treated with IMU. In conclusion, at appropriate concentrations, each of the four preservatives modulates the apoptotic machinery by a caspase-dependent mechanism. Thus, apoptosis could be a good parameter to evaluate the cytoxicity of these chemical compounds.
Skin, Eye and Respiratory Irritations:
A skin and severe eye irritant.
Drug Warnings:
Peritonitis is the established term for infective inflammation of the peritoneum, whereas serositis generally refers to aseptic inflammation of a serous cavity, including the peritoneum. Serositis may be metabolic, viral, autoimmune, drug induced, genetic, allergic or granulomatous, or due to chemical antiseptics. In ...gynecological department, 4 patients had peritonitis and ascites after laparotomy. Based on the investigation... the solution used for peritoneal lavage (0.1% octenidine dihydrochloride and 2% phenoxyethanol) played a role in the tissue toxicity that caused chemical serositis with effusion.
Probable Routes of Human Exposure:
According to the 2006 TSCA Inventory Update Reporting data, the number of persons reasonably likely to be exposed in the industrial manufacturing, processing, and use of 2-phenoxyethanol is 1000 or greater; the data may be greatly underestimated(1).
NIOSH (NOES Survey 1981-1983) has statistically estimated that 111,040 workers (46,637 of these were female) were potentially exposed to 2-phenoxyethanol in the US(1). Occupational exposure to the 2-phenoxyethanol occurs through inhalation of vapor and dermal contact. Its use as solvent for inks, resins and cellulose acetate and its use as a perfume fixative could expose the general population through dermal contact and inhalation of vapor(SRC).
Emergency Medical Treatment:
Emergency Medical Treatment:
| EMT Copyright Disclaimer: |
| The information contained in the Truven Health Analytics Inc. products is intended as an educational aid only. All treatments or procedures are intended to serve as an information resource for physicians or other competent healthcare professionals performing the consultation or evaluation of patients and must be interpreted in view of all attendant circumstances, indications and contraindications. The use of the Truven Health Analytics Inc. products is at your sole risk. These products are provided "as is" and "as available" for use, without warranties of any kind, either express or implied. Truven Health Analytics Inc. makes no representation or warranty as to the accuracy, reliability, timeliness, usefulness or completeness of any of the information contained in the products. Additionally, Truven Health ANALYTICS INC. makes no representation or warranties as to the opinions or other service or data you may access, download or use as a result of use of the Truven Health ANALYTICS INC. products. All implied warranties of merchantability and fitness for a particular purpose or use are hereby excluded. Truven Health Analytics Inc. does not assume any responsibility or risk for your use of the Truven Health Analytics Inc. products. The following Overview, *** GLYCOL ETHERS ***, is relevant for this HSDB record chemical. |
| Life Support: |
o This overview assumes that basic life support measures
have been instituted. |
| Clinical Effects: |
0.2.1 SUMMARY OF EXPOSURE
0.2.1.1 ACUTE EXPOSURE
A) USES: This management reviews the general toxicology of
ethylene glycol monoalkyl ethers. These compounds are
referred to as glycol ethers (eg, ethylene glycol butyl
ether (EGBE), ethylene glycol methyl ether (EGME),
ethylene glycol ethyl ether (EGEE)), ethers of ethylene
glycol, or by their respective alkoxyalcohol (eg,
butoxyethanol, ethoxyethanol, methoxyethanol)
designation. Glycol ethers are used and found in many
solvent mixtures. These solvents are commonly used as
cleaning products, automotive fluids, lacquers, varnish
removers, leather treatment products, dyeing and
printing of textiles, and anti-icing agents for
aviation fuels.
B) TOXICOLOGY: Toxic effects are likely due to both the
parent compound and metabolites, but the mechanisms of
toxicity are unknown. Following excessive exposure,
these agents may cause central nervous system, renal,
and hematologic toxicity. It remains speculation that
glycol ethers may undergo cleavage of the ether bond to
produce ethylene glycol with subsequent metabolism to
oxalate. Acidosis might occur secondary to metabolism
via alcohol dehydrogenase to alkoxyacids.
C) EPIDEMIOLOGY: Acute poisoning is relatively rare but
glycol ethers are used widely in industrial settings
and chronic exposure may occur in many workers among
certain industries. Life-threatening effects are rare,
but have been reported following large, deliberate
ingestion.
D) WITH POISONING/EXPOSURE
1) MILD TO MODERATE TOXICITY: Most inadvertent ingestions
of household products containing glycol ethers are
asymptomatic. With acute inhalational exposure, eye
and upper respiratory tract irritation may occur.
Other symptoms that may occur include CNS symptoms
such as headache, drowsiness, lethargy, fatigue,
dizziness, weakness, staggered gait, and tremor. In
chronic inhalation exposures, there have been reports
of gynecologic disorders (benign neoplasms, cervical
erosions, menstrual disorders), decreased sperm
counts, leukopenia, mild anemia or granulocytopenia,
and kidney injury (hematuria, albuminuria). Direct eye
contact exposure may cause immediate pain, corneal and
conjunctival irritation, and tearing that usually
clears after one day. Hypocalcemia and hypokalemia
have also been observed.
2) SEVERE TOXICITY: In large and intentional exposures,
CNS depression, metabolic acidosis, renal injury,
hypotension, seizures, acute lung injury, mild
elevations in liver enzymes, hemolytic anemia,
thrombocytopenia, and disseminated intravascular
coagulation have been reported. ARDS was reported in
one case after ingestion of 500 mL of 9.1% EGBE.
Severe hypotension and ventricular dysrhythmias are
rare effects of large ingestions of EGBE. Hemorrhagic
gastritis and fatty degeneration of the liver were
seen in a case of fatal poisoning with methyl ether.
0.2.4 HEENT
0.2.4.1 ACUTE EXPOSURE
A) Eye irritation is generally slight, but may be severe
with the propyl ether.
0.2.20 REPRODUCTIVE HAZARDS
A) Teratogenic effects have been observed at maternally
non-toxic doses for the methyl ether and ethyl ether of
ethylene glycol.
0.2.21 CARCINOGENICITY
0.2.21.1 IARC CATEGORY
A) IARC Carcinogenicity Ratings for CAS111-76-2
(International Agency for Research on Cancer (IARC),
2016; International Agency for Research on Cancer,
2015; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2010; IARC Working Group
on the Evaluation of Carcinogenic Risks to Humans,
2010a; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2008; IARC Working Group
on the Evaluation of Carcinogenic Risks to Humans,
2007; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2006; IARC, 2004):
1) IARC Classification
a) Listed as: 2-Butoxyethanol
b) Carcinogen Rating: 3
1) The agent (mixture or exposure circumstance) is not
classifiable as to its carcinogenicity to humans.
This category is used most commonly for agents,
mixtures and exposure circumstances for which the
evidence of carcinogenicity is inadequate in humans
and inadequate or limited in experimental animals.
Exceptionally, agents (mixtures) for which the
evidence of carcinogenicity is inadequate in humans
but sufficient in experimental animals may be placed
in this category when there is strong evidence that
the mechanism of carcinogenicity in experimental
animals does not operate in humans. Agents, mixtures
and exposure circumstances that do not fall into any
other group are also placed in this category.
B) IARC Carcinogenicity Ratings for CAS109-86-4
(International Agency for Research on Cancer (IARC),
2016; International Agency for Research on Cancer,
2015; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2010; IARC Working Group
on the Evaluation of Carcinogenic Risks to Humans,
2010a; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2008; IARC Working Group
on the Evaluation of Carcinogenic Risks to Humans,
2007; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2006; IARC, 2004):
1) Not Listed
C) IARC Carcinogenicity Ratings for CAS110-80-5
(International Agency for Research on Cancer (IARC),
2016; International Agency for Research on Cancer,
2015; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2010; IARC Working Group
on the Evaluation of Carcinogenic Risks to Humans,
2010a; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2008; IARC Working Group
on the Evaluation of Carcinogenic Risks to Humans,
2007; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2006; IARC, 2004):
1) Not Listed
D) IARC Carcinogenicity Ratings for CAS109-59-1
(International Agency for Research on Cancer (IARC),
2016; International Agency for Research on Cancer,
2015; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2010; IARC Working Group
on the Evaluation of Carcinogenic Risks to Humans,
2010a; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2008; IARC Working Group
on the Evaluation of Carcinogenic Risks to Humans,
2007; IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, 2006; IARC, 2004):
1) Not Listed
0.2.22 GENOTOXICITY
A) Ethylene glycol n-butyl ether and the aldehyde
metabolite of ethylene glycol monomethyl ether
(methoxyacetaldehyde) displayed mutagenic potency in the
Salmonella typhimurium assay. In contrast, EGME, EGEE,
EALD, BALD and the acid metabolites gave negative
responses with all strains of Salmonella typhimurium
used in the assay (Hoflack et al, 1995). |
| Laboratory: |
A) Monitor vital signs and mental status.
B) Monitor CBC, serum electrolytes, liver enzymes, renal
function, urinalysis, and urine output in symptomatic
patients or after a significant ingestion.
C) Monitor arterial blood gases for worsening metabolic
acidosis.
D) Serum ethanol, methanol, and ethylene glycol
concentrations are theoretically useful in assessing
concurrent ingestions or metabolites of glycol ethers.
E) It remains speculation that glycol ethers may undergo
cleavage of the ether bond to produce ethylene glycol,
with subsequent metabolism to oxalate. However, elevated
urinary oxalate concentrations and urinary oxalate
crystals have been reported after human overdose. |
| Treatment Overview: |
0.4.2 ORAL EXPOSURE
A) MANAGEMENT OF MILD TO MODERATE TOXICITY
1) Treatment is symptomatic and supportive.
B) MANAGEMENT OF SEVERE TOXICITY
1) Treatment is symptomatic and supportive. Fluid
resuscitation and hemodialysis may be needed for severe
acid-base abnormality. Treat severe acidosis (pH less
than 7.1) with intravenous sodium bicarbonate. Begin
with 1 to 2 mEq/kg in adults and 1 mEq/kg in children,
repeat every 1 to 2 hours as required. Treat severe
hypotension with IV 0.9% NaCl at 10 to 20 mL/kg. Add
dopamine or norepinephrine if unresponsive to fluids.
Alcohol dehydrogenase inhibition with ethanol or
fomepizole has been used, however, its effectiveness is
unclear. There are reports of progression of acidosis
following fomepizole treatment. Hemodialysis for severe
and persistent symptoms has shown to improve clinical
and metabolic status.
C) DECONTAMINATION
1) PREHOSPITAL: Gastrointestinal decontamination is not
indicated due to rapid absorption and the risk of CNS
depression and aspiration after large ingestion.
Irrigate exposed eyes after ocular exposure. Remove
contaminated clothing and wash exposed skin. Remove
patients with inhalational exposure to fresh air.
Administer oxygen if respiratory irritation develops.
2) HOSPITAL: Irrigate exposed eyes after ocular exposure.
Remove contaminated clothing and wash exposed skin. In
patients with inhalational exposure, administer oxygen
if respiratory irritation develops. ACTIVATED CHARCOAL:
Not routinely recommended. The risk of aspiration is
high and activated charcoal likely does not bind these
substances well. GASTRIC ASPIRATION: As these products
are generally liquid, early nasogastric aspiration may
be useful for very large, very recent ingestions if the
patient is alert or the airway is protected.
D) AIRWAY MANAGEMENT
1) Endotracheal intubation may be required if significant
CNS depression occurs.
E) ANTIDOTE
1) There is no specific antidote for the treatment of
glycol ethers exposure. Although fomepizole and ethanol
are not approved for use in glycol ether poisoning,
they may be effective in preventing formation of the
acid metabolites and lessening toxicity. They should be
considered for large ingestions in patients developing
metabolic acidosis or renal insufficiency. FOMEPIZOLE
VS ETHANOL: Fomepizole is easier to use clinically,
requires less monitoring, and does not cause CNS
depression or hypoglycemia. Ethanol requires continuous
administration and frequent monitoring of serum ethanol
and glucose levels, and may cause CNS depression and
hypoglycemia (especially in children). The drug cost
associated with ethanol use is generally much lower
than with fomepizole; however, other costs associated
with ethanol use (continuous intravenous infusion,
hourly blood draws and ethanol levels, possibly greater
use of hemodialysis) may make the costs more
comparable.
a) FOMEPIZOLE: Fomepizole is administered as a 15 mg/kg
loading dose, followed by four bolus doses of 10 mg/kg
every 12 hours. If therapy is needed beyond this 48
hour period, the dose is then increased to 15 mg/kg
every 12 hours for as long as necessary. Fomepizole is
also effectively removed by hemodialysis; therefore,
doses should be repeated following each round of
hemodialysis.
b) ETHANOL: Ethanol is given to maintain a serum ethanol
concentration of 100 to 150 mg/dL. This can be
accomplished by using a 5% to 10% ethanol solution
administered IV through a central line. Intravenous
therapy dosing, which is preferred, is 0.8 g/kg as a
loading dose (8 mL/kg of 10% ethanol) administered
over 20 to 60 minutes as tolerated, followed by an
infusion rate of 80 to 150 mg/kg/hr (for 10% ethanol,
0.8 to 1.3 mL/kg/hr for a nondrinker; 1.5 mL/kg/hr for
a chronic alcoholic). During hemodialysis, either add
ethanol to the dialysate to achieve 100 mg/dL
concentration or increase the rate of infusion during
dialysis (for 10% ethanol, 2.5 to 3.5 mL/kg/hr). Blood
ethanol concentrations must be monitored hourly and
the infusion adjusted accordingly.
F) ENHANCED ELIMINATION
1) There is no clinical experience; however, hemodialysis
is indicated for severe acid-base and/or
fluid-electrolyte abnormalities despite conventional
therapy, or renal failure. It is unknown if glycol
ethers are removed by hemolysis.
G) PATIENT DISPOSITION
1) HOME CRITERIA: Asymptomatic, inadvertent ingestions
(taste or sip of a household product) , dermal or
inhalation exposures may be managed at home.
2) OBSERVATION CRITERIA: All symptomatic patients, or
those with large or intentional ingestions should be
referred to a healthcare facility for evaluation.
Patients with significant ingestions should be observed
for at least 24 hours. Delayed symptom onset of 8 to 18
hours has been seen following ingestion of some glycol
ethers. Symptomatic patients with less significant
exposures may be sent home if their symptoms are
clearly improving and discharge is clinically
indicated.
3) ADMISSION CRITERIA: Patients with worsening symptoms
should be admitted to the hospital for further
treatment and evaluation. Admission to ICU may be
required based on the severity of symptoms. Criteria
for discharge includes clearly improving symptoms in
patients who are clinically stable.
4) CONSULT CRITERIA: Consult a medical toxicologist or
Poison Center for assistance in managing patients with
severe toxicity or in whom diagnosis is unclear.
Consult a nephrologist for severe acidosis, renal
issues, or potential hemodialysis. Consultation with a
critical care physician may be needed in cases of
severe toxicity.
H) PITFALLS
1) Failure to recognize co-ingestions, or ingestion of
another toxic alcohol. Failure to recognize progression
and worsening of symptoms. Discharging patient after
only 6 hours of observation following a significant
ingestion. Delayed symptom onset of 8 to 18 hours has
been seen following ingestion of some glycol ethers.
I) TOXICOKINETICS
1) Glycol ethers are rapidly absorbed orally, and can be
absorbed vial prolonged dermal and inhalational
exposures. Distribution is rapid and extensive. Many of
the glycol monoalkyl ethers have been shown to be
oxidized by alcohol dehydrogenase in the liver to their
respective alkoxyacetic acid. The major metabolites
appear to be the acid derivatives and glycine
conjugates. Butoxyacetic acid, methoxyacetic acid,
ethoxyacetic acid, and isopropoxyacetic acid, are the
major metabolites of the butyl ether, methyl ether,
ethyl ether, and isopropyl ether, respectively. There
is no direct evidence that glycol ethers undergo
cleavage of the ether bond to produce ethylene glycol,
with subsequent metabolism to oxalate. The elimination
half-life of butoxyacetic acid in 5 volunteers exposed
to 20 ppm of 2-butoxyethanol ranged from 1.7 to 9.6
hours. Urinary ethoxyacetic acid was detected in urine
within the first hour of human inhalations, with
continuing increases until 3 to 4 hours post exposure,
when a subsequent exponential decline in urinary
ethoxyacetic acid occurred, with an elimination half
life of 21 to 24 hours.
J) DIFFERENTIAL DIAGNOSIS
1) Consider exposures to other toxic alcohols or glycols:
methanol, ethylene glycol, diethylene glycol,
isopropanol, and ethanol.
0.4.3 INHALATION EXPOSURE
A) Move patient from toxic environment to fresh air.
Monitor for respiratory distress and administer oxygen
as needed. Treat bronchospasms with inhaled
beta-2-agonists.
0.4.4 EYE EXPOSURE
A) Remove contact lenses and irrigate exposed eyes with
normal saline or water for at least 15 minutes. If
symptoms continue after irrigation, an ophthalmologic
examination is indicated.
0.4.5 DERMAL EXPOSURE
A) OVERVIEW
1) Remove contaminated clothing and wash exposed areas
thoroughly with soap and water. |
| Range of Toxicity: |
A) TOXICITY: Adults have developed severe toxicity after
ingestion of 30 to 100 mL. Depression of CNS and
hematopoietic effects were reported with chronic
exposures to 25 to 76 ppm of glycol ethers.
B) EGBE: Severe toxicity has been described in adults who
ingested 30 to 63.5 mL of pure EGBE. Children ingesting
small amounts (less than 10 mL) of dilute household
products (less than 10% EGBE) generally do not develop
evidence of poisoning. The threshold limit value weighted
average for an 8 hour shift (TLV-TWA) is 20 ppm, 700 ppm
is considered immediately dangerous to life and health.
C) EGME: Renal failure has occurred with ingestion of 100 mL
in adults. Inhalation of 60 ppm may produce CNS and
hematologic effects. Death after 240 mL of EGME has been
observed. The 8-hour threshold limit value (TLV)
time-weighted average (TWA) is 0.1 ppm; 200 ppm is
considered immediately dangerous to life and health.
D) EGEE: An exposure of 500 parts per million (ppm) is
considered immediately dangerous to life and health. It
is not significantly irritating to skin, mildly
irritating to eyes and mucous membranes, and considered
low toxicity after dermal exposure. An adult ingestion of
40 mL caused significant symptoms including CNS
depression and respiratory issues. |
Antidote and Emergency Treatment:
/SRP:/ Immediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on the left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. /Poisons A and B/
/SRP:/ Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if needed. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 mL/kg up to 200 mL of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool ... . Cover skin burns with dry sterile dressings after decontamination ... . /Poisons A and B/
/SRP:/ Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Positive-pressure ventilation techniques with a bag valve mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Consider administering a beta agonist such as albuterol for severe bronchospasm ... . Monitor cardiac rhythm and treat arrhythmias as necessary ... . Start IV administration of D5W /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's if signs of hypovolemia are present. For hypotension with signs of hypovolemia, administer fluid cautiously. Watch for signs of fluid overload ... . Treat seizures with diazepam or lorazepam ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Poisons A and B/
Animal Toxicity Studies:
Non-Human Toxicity Excerpts:
/LABORATORY ANIMALS: Acute Exposure/ IN UNDILUTED FORM, /2-phenoxyethanol/ IS SEVERELY DAMAGING TO EYES OF RABBITS. WHEN DILUTED TO 5%, IT CAUSED ONLY MILD IRRITATION OF CONJUNCTIVAL MEMBRANES. RATS TOLERATED, WITHOUT APPARENT ADVERSE EFFECTS, 0NE 7 HR EXPOSURE TO VAPORS SATURATED AT 100 DEG C AND COOLED TO ROOM TEMP.
/LABORATORY ANIMALS: Subchronic or Prechronic Exposure/ Studies were conducted to characterize the hemolytic effects of / ethylene glycol phenyl ether / EGPE in rabbits following oral ...exposure... Gavage administration of EGPE to female New Zealand White rabbits at 100, 300, 600, or 1000 mg/kg/day for up to 10 consecutive days (one dose/day) resulted in a dose-related intravascular hemolytic anemia. The hemolytic anemia was characterized by decreased RBC count, hemoglobin concentration, packed cell volume, hemoglobinuria, splenic congestion, renal tubule damage, and a regenerative erythroid response in the bone marrow. The hemolytic anemia was observed without alterations in RBC glutathione or methemoglobin.
/LABORATORY ANIMALS: Subchronic or Prechronic Exposure/ Studies were conducted to characterize the hemolytic effects of /ethylene glycol phenyl ether/ EGPE in rabbits following ... dermal exposure...In a 90-day dermal study in which EGPE was applied to the skin of male and female New Zealand White rabbits 6 hr/day, 5 days/week, at doses up to 500 mg/kg/day, there was no indication of a hemolytic response. The only treatment-related effects were sporadic occurrences of slight erythema and scaling of skin at the site of test material application in high dose group male and female rabbits. However, erythema and scaling were not associated with gross or histopathologic changes and were not considered toxicologically significant.
/LABORATORY ANIMALS: Developmental or Reproductive Toxicity/ Pregnant New Zealand white rabbits were treated dermally with 300, 600, or 1000 mg/kg/day of undiluted 2-phenoxyethanol on days 6 thru 18 of gestation (25 animals per dose group). 2-Phenoxyethanol was toxic to the dams (maternal death) at the 600 and 1000 mg/kg doses. No adverse effects on pregnancy rate, resorptions, or fetal body measurements were observed at any dose. 2-Phenoxyethanol did not cause malformations in the fetuses as compared with controls.
/LABORATORY ANIMALS: Developmental or Reproductive Toxicity/ Acute toxicity tests were performed on aquarium fish Danio rerio, which is considered to be one of the model organisms most commonly used in toxicity testing. The semi-static method according to OECD No. 203 (Fish acute toxicity test) was used for testing juvenile fish. Embryo toxicity tests were performed in zebrafish embryos (D. rerio) in compliance with the OECD No. 212 methodology (Fish, short-term toxicity test on embryo and sac-fry stages). The results obtained (the number of dead individuals at particular test concentrations) were subjected to a probit analysis using the EKO-TOX 5.2 programme in order to determine LC50 clove oil and 2-phenoxyethanol values. The statistical significance of the difference between LC50 values in juvenile and embryonic stages of D. rerio was tested using the Mann-Whitney non-parametric test implemented in the Unistat 5.1 programme.The LC50 clove oil mean value was 18.8 +/- 5.52 mg /per/ L in juvenile D. rerio, and 15.64 +/- 3.30 mg /per/ L in embryonic stages of D. rerio. The LC50 2-phenoxyethanol mean value was 338.22 +/- 15.22 mg /per/ L in juvenile D. rerio, whereas in embryonic stages of D. rerio it was 486.35 +/- 25.53 mg /per/ L. The study proved statistically significantly higher (p<0.01) sensitivity in juvenile fish to 2-phenoxyethanol compared to the embryonic stages. Acute toxicity values of clove oil for juvenile and embryonic stages were comparable.
/ALTERNATIVE and IN VITRO TESTS/ The growth adaptability to increasing concentration of the biocide 2-phenoxyethanol (PE) was determined in Pseudomonas aeruginosa PAO1 (P.a.) as part of efforts to understand and control the biocide tolerance and its effect on cross-resistance to other biocides and resistance to antibiotics. After repeated subculturing in media containing increasing sub-minimum-inhibitory PE concentration, P.a. exhibited an adaptive resistance indicated by two-fold increase in MIC at the 10th passage. The resistance was stable and remained after passaging the strain in further 7 successive passages in PE-free growth media. The strain showed cross-resistance towards dissimilar biocides and displayed increased susceptibility to antibiotics, which was not influenced by the presence of the efflux inhibitor 'carbonyl cyanide m-chlorophenyl hydrazone'. Outer membranes of adapted strain showed altered protein profile when examined by SDS-PAGE.
Ecotoxicity Excerpts:
/AQUATIC SPECIES/ PHENOXYETHANOL SHOWED A TOXIC EFFECT ON FERTILIZATION WHEN THE CONCN IN THE INSEMINATION DILUTENT WAS 0.05%. THIS EFFECT WAS LIMITED TO THE SPERM. THE ANESTHETIC DID NOT SEEM TO AFFECT THE EGG. THEREFORE, CAUTION SHOULD BE EXERCISED WHEN PHENOXYETHANOL IS USED TO IMMOBILIZE FISH DURING SPAWN TAKING.
/AQUATIC SPECIES/ 2-PHENOXYETHANOL (0.1-0.5 ML/L) SEDATED OR ANESTHETIZED FISH WITHIN MINUTES WHEN THE ANIMALS WERE IMMERSED IN THE AGENT. THE FISH RECOVERED RAPIDLY FOLLOWING REMOVAL FROM THE ANESTHETIC SOLN.
/AQUATIC SPECIES/ /Fathead Minnows were exposed to 2-Phenoxyethanol at nominal concentrations of 0, 68, 113, 188, 313, 522 mg/L for up to 96 hr./ Fish in the second highest concentration were immediately affected but began to respond to tap 12 hr. They did not school for the remainder of the test. Affected fish stopped schooling, became hypoactive on the tank bottom, then lost equilibrium prior to death.
/AQUATIC SPECIES/ THE APPROX TIMES TO 50% MORTALITY OF JUVENILE RAINBOW TROUT (SALMO GAIRDNERI) EXPOSED TO 2-PHENOXYETHANOL AT 0.75, 0.50, & 0.25 ML/L WERE 10.7 MINUTES, 26.3 MINUTES, & 3.7 HR, RESPECTIVELY. THE AVERAGE IMMOBILIZATION TIMES AT THE ABOVE 3 CONCN WERE 2, 3, & 4 MINUTES, RESPECTIVELY, & RECOVERY TIMES WERE APPROX 14, 9, & 6 MINUTES, RESPECTIVELY, FOR FISH THAT SURVIVED. 2-PHENOXYETHANOL THUS APPEARS TO BE A SUITABLE ANESTHETIC FOR JUVENILE SALMONIDS BUT ONLY FOR LIMITED DURATIONS, ESPECIALLY AT HIGHER CONCN.
National Toxicology Program Studies:
Ethylene Glycol Monophenyl Ether (EGPE) ... was tested for reproductive toxicity in Swiss CD-1 mice using the RACB protocol. ... Data collected on body weights, clinical signs, & food/water consumption during the dose-range-finding segment (Task 1) were used to set concn for the main study (Task 2) at 0.0, 0.25, 1.25, 2.5% in feed. These concn produced calculated consumption estimates of nearly equal to 375, 1875, & 3700 mg/kg/day. There were no effects on body weight during the continuous breeding phase of the study. Two control mice died, & one mouse & two mice died in the middle & high dose groups, respectively. All pairs of mice in each group had at least 1 litter. There was no reduction in the mean number of litters/pair. The middle dose group had 5.00 litters/pair, while the control had a mean of 4.84; this difference was statistically significant, but biologically insignificant. The high dose group had 19% fewer live pups/litter than controls; the live pup weight (adjusted for litter size) was reduced by 4% & 10% in the middle & high dose groups, respectively. Because of the reduction in pup number, a crossover mating trial was conducted, using one treated partner & one control partner. A separate group of re-randomized controls served as concurrent controls for this task. While there were no alterations in mating or fertility indices or in the number of live pups/litter seen in groups with a treated partner, live pup weight adjusted for litter size was reduced by 12% in the control male x 2.5% EGPE female group. Thus, there was a clear effect in treated females, but one probably related to developmental toxicity, rather than female fertility per se. The control & high dose F0 mice were killed & necropsied. The treated males weighed 6% less than their controls, while their absolute liver weight was 14% greater. Female body weight was unchanged by EGPA, but absolute liver weight was increased by 55%. No other organ weights were affected. Sperm indices (% motile, epididymal concn, morphology) were unaffected by EGPE treatment at 2.5%. The last F1 litter from all dose levels in Task 2 was reared by the dams to weaning, & then dosed with EGPE at the same concn provided to their parents. There was reduced body weight gain to weaning: the middle & high dose groups weighed 25% & 58% less than controls at weaning on /postnatal day/ 21; on /postnatal day/ 74, the weight differences were 11% & 17%, respectively. Mortality was also increased in the middle & high dose groups from weaning to mating at /postnatal day/ 74. This was most pronounced in the high dose group: of the 56 pups weaned in this group, only a total of 6 survived to mating at /postnatal day/ 74. This provided too few animals to analyze, & this group was omitted from the rest of the study. At the mating of the second generation, there was no treatment-related effect on F2 pup number or sex ratio. F2 pup weight adjusted for litter size was reduced in the 1.25% group by 7%. After the delivery of the F2 pups, the control & 1.25% group F1 mice were killed & necropsied. The 1.25% EGPE mice weighed 13% less than controls, their absolute testis weight was 16% less, & relative seminal vesicles weight was 14% less than controls. The 1.25% EGPE females weighed 7% less than controls; there were no adjusted weight changes in the treated females. There were no treatment-related alterations in epididymal sperm concn, motility, or morphology. In summary, EGPE produced significant reproductive & developmental toxicity at doses that increased liver weight in treated F0 mice. EGPE caused significant toxicity in growing animals, as evidenced by the reduced body weight in neonates in Tasks 2, 3, & 4, & the large incr in post-natal lethality as the F1 animals grew to the age of mating.
Non-Human Toxicity Values:
LD50 Mouse ip 872 mg/kg bw
LD50 Mouse ip ca 333 mg/kg bw
LD50 Guinea pig dermal >22180 mg/kg bw
LD50 Rabbit dermal >5000 mg/kg bw
LD50 Rabbit dermal 2250 mg/kg bw
LD50 Rabbit dermal 3815 mg/kg bw
LD50 Rabbit dermal 3660 mg/kg bw
LD50 Rabbit dermal 5545 mg/kg bw
LD50 Rabbit dermal >2218 mg/kg bw
LD50 Rat dermal 2300-3800 mg/kg bw
LD50 Rat dermal 14422 mg/kg bw
LD50 Rat oral 2728 mg/kg bw
LD50 Rat oral ca 7500 mg/kg bw
LD50 Rat oral 4013 mg/kg bw
LD50 Rat oral 2937 mg/kg bw
LD50 Rat oral 2000 mg/kg bw
LD50 Rat oral 2580 mg/kg bw
LD50 Rat oral 2000-3000 mg/kg bw
LD50 Rat oral ca 1345 mg/kg bw
LD50 Rat oral 3100 mg/kg bw
LD50 Rat oral 1440 mg/kg
LD50 Rat oral 3400 mg/kg
LD50 Rat oral 1400-2580 mg/kg
LD50 Rat oral 5550 mg/kg
LD50 Rat oral 1260 mg/kg
LD50 MOUSE ORAL 16,500 MG/KG PLASTIAZAN-41 (ETHYLENE GLYCOL PHENYL ETHER)
LD50 Rabbit skin 5000 mg/kg
Ecotoxicity Values:
EC50; Species: Pimephales promelas (fathead minnow); Conditions: flow-through bioassay with measured concentrations, 26.6 deg C, dissolved oxygen 6.0 mg/L, hardness 45.0 mg/L calcium carbonate, alkalinity 42.0 mg/L calcium carbonate, and pH 7.62; Concentration: 344 mg/L for 96 hr (confidence limit 337-352 mg/L); Effect: loss of equilibrium
LC50; Species: Pimephales promelas (fathead minnow); Conditions: flow-through bioassay with measured concentrations, 26.6 deg C, dissolved oxygen 6.0 mg/L, hardness 45.0 mg/L calcium carbonate, alkalinity 42.0 mg/L calcium carbonate, and pH 7.62; Concentration: 344 mg/L for 96 hr (confidence limit 337-352 mg/L)
TSCA Test Submissions:
Teratogenicity was evaluated in pregnant New Zealand White rabbits (25/group) dermally exposed to 2-phenoxyethanol at treatment levels of 0, 300, 600, and 1000 mg/kg/day on gestation days (GD) 6-18. Surviving animals were sacrificed on GD 28. Significant differences were observed between treated and control animals in the following: slight to moderate reddening of the skin at the application site (all treated animals), maternal mortality with dead animals exhibiting dark-colored urine in the bladder, darkened kidneys, evidence of anorexia (hairball in stomach), superficial erosions, ulcers, and/or hemorrhages in the gastric mucosa, decreased feed and fecal material in the intestines, severely decreased red blood cell counts and packed cell volume, increased reticulocytes (evidence of a regenerative hemolytic anemia and the animals were jaundiced (high- and mid-dose groups). No significant differences were observed between treated and control animals in the following (mid- and low-dose groups unless otherwise noted): maternal body weights or weight gain, liver weights, pregnancy rates, resorptions, preimplantation losses, fetal sex ratio or body measurements, and incidence of fetal malformations or alterations in gross morphology, internal organs or skeletal system. No statistical evaluations were performed on the five high-dose group rabbits which survived until GD 28.
Metabolism/Pharmacokinetics:
Metabolism/Metabolites:
Once hydrolyzed, 2-phenoxyethanol is rapidly absorbed and oxidized to phenoxyacetic acid ...
YIELDS PHENOL IN CONIOPHORA, IN PLEUROTUS, & IN POLYSTICTUS ... . /FROM TABLE/
The toxicity of glycol ethers is associated with their oxidation to the corresponding aldehyde and alkoxyacetic acid by cytosolic alcohol dehydrogenase (ADH; EC 1.1.1.1.) and aldehyde dehydrogenase (ALDH; 1.2.1.3). Dermal exposure to these compounds can result in localised or systemic toxicity including skin sensitisation and irritancy, reproductive, developmental and hematological effects. It has previously been shown that skin has the capacity for local metabolism of applied chemicals. Therefore, there is a requirement to consider metabolism during dermal absorption of these compounds in risk assessment for humans. Cytosolic fractions were prepared from rat liver, and whole and dermatomed skin by differential centrifugation. Rat skin cytosolic fractions were also prepared following multiple dermal exposure to dexamethasone, ethanol or 2-butoxyethanol (2-BE). The rate of ethanol, 2-ethoxyethanol (2-EE), ethylene glycol, 2-phenoxyethanol (2-PE) and 2-BE conversion to alkoxyacetic acid by ADH/ALDH in these fractions was continuously monitored by UV spectrophotometry via the conversion of NAD+ to NADH at 340 nm. Rates of ADH oxidation by rat liver cytosol were greatest for ethanol followed by 2-EE >ethylene glycol >2-PE >2-BE. However, the order of metabolism changed to 2-BE >2-PE >ethylene glycol >2-EE >ethanol using whole and dermatomed rat skin cytosolic fractions, with approximately twice the specific activity in dermatomed skin cytosol relative to whole rat skin. This suggests that ADH and ALDH are localised in the epidermis that constitutes more of the protein in dermatomed skin than whole skin cytosol. Inhibition of ADH oxidation in rat liver cytosol by pyrazole was greatest for ethanol followed by 2-EE >ethylene glycol >2-PE >2-BE, but it only inhibited ethanol metabolism by 40% in skin cytosol. Disulfiram completely inhibited alcohol and glycol ether metabolism in the liver and skin cytosolic fractions. Although ADH1, ADH2 and ADH3 are expressed at the protein level in rat liver, only ADH1 and ADH2 are selectively inhibited by pyrazole and they constitute the predominant isoforms that metabolise short-chain alcohols in preference to intermediate chain-length alcohols. However, ADH1, ADH3 and ADH4 predominate in rat skin, demonstrate different sensitivities to pyrazole, and are responsible for metabolising glycol ethers. ALDH1 is the predominant isoform in rat liver and skin cytosolic fractions that is selectively inhibited by disulfiram and responds to the amount of aldehyde formed by the ADH isoforms expressed in these tissues. Thus, the different affinity of ADH and ALDH for alcohols and glycol ethers of different carbon-chain length may reflect the relative isoform expression in rat liver and skin. Following multiple topical exposure, ethanol metabolism increased the most following ethanol treatment, and 2-BE metabolism increased the most following 2-BE treatment. Ethanol and 2-BE may induce specific ADH and ALDH isoforms that preferentially metabolise short-chain alcohols (i.e. ADH1, ALDH1) and longer chain alcohols (i.e. ADH3, ADH4, ALDH1), respectively. Treatment with a general inducing agent such as dexamethasone enhanced ethanol and 2-BE metabolism suggesting induction of multiple ADH isoforms.
Studies were conducted... to evaluate the in vitro hemolytic potential of / ethylene glycol phenyl ether/ EGPE and its major metabolite using rabbit red blood cells (RBC). Phenoxyacetic acid (PAA) was identified as a major blood metabolite of EGPE. In vitro exposure of female rabbit erythrocytes indicated EGPE to be considerably more hemolytic than PAA.
Absorption, Distribution & Excretion:
... An entire oral dose of 11 mg of unlabelled 2-phenoxyethanol was accounted for in the urine of one healthy male volunteer as 2-phenoxyacetic acid. Most of the acid was excreted unconjugated.
The fate of 2-phenoxyethanol in rats and humans has been investigated. More than 90% of an oral dose of 16, 27 or 160 mg/kg bw of (2-(14)C)phenoxyethanol given to male Colworth rats by gavage was excreted in the urine within 24 hr. A female rat also excreted about 90% of a dose of 27 mg/kg bw in the urine within 24 hr. Approximately 2 and 1.3% of the ingested dose was recovered from expired air of female and male rats, respectively. The rate of intestinal absorption was rapid, with 60-70% of the excreted (14)C detected at 3 hr and > 95% of the total 4-day urinary (14)C detected within the first 24 hr. Trace amounts of radioactivity were detected in feces. Four days after dosing, only trace amounts of radioactivity remained in the carcass, primarily in the liver (< 0.2% of the dose), fat and muscle. At 4 days, the (14)C concentration in blood was only 0.001.
... NOT READILY ABSORBED THROUGH THE SKIN IN ACUTELY TOXIC AMT.
2-PHENOXYETHANOL (0.1-0.5 ML/L) SEDATED OR ANESTHETIZED FISH WITHIN MINUTES WHEN THE ANIMALS WERE IMMERSED IN THE AGENT. WHEN ADMIN IN THIS WAY, THE ANESTHETIC WAS ABSORBED INTO THE BLOOD STREAM THROUGH THE GILL LAMELLAE.
Pharmacology:
Therapeutic Uses:
Phenoxyethanol (PE) is a preservative added to cosmetics and pharmaceuticals such as antibiotic ointments and solutions, ear-drops, and vaccines.
Anti-Infective Agents, Local; Anesthetics
Phenoxyethanol has antibacterial properties and is effective against strains of Pseudomonas aeruginosa even in the presence of 20% serum. It is less effective against Proteus vulgaris, other Gram-negative organisms, and Gram-positive organisms. It has been used as a preservative at a concentration of 1%. A wider spectrum of antimicrobial activity is obtained with preservative mixtures of phenoxyethanol and hydroxybenzoates. Phenoxyethanol may be used as a 2.2% solution or a 2% cream for the treatment of superficial wounds, burns, or abscesses infected by Pseudomonas aeruginosa. In skin infection derivatives of phenoxyethanol are used with either cyclic acid or zinc undecenoate.
TOPICAL ANTISEPTIC
Drug Warnings:
Peritonitis is the established term for infective inflammation of the peritoneum, whereas serositis generally refers to aseptic inflammation of a serous cavity, including the peritoneum. Serositis may be metabolic, viral, autoimmune, drug induced, genetic, allergic or granulomatous, or due to chemical antiseptics. In ...gynecological department, 4 patients had peritonitis and ascites after laparotomy. Based on the investigation... the solution used for peritoneal lavage (0.1% octenidine dihydrochloride and 2% phenoxyethanol) played a role in the tissue toxicity that caused chemical serositis with effusion.
Environmental Fate & Exposure:
Environmental Fate/Exposure Summary:
2-Phenoxyethanol's production and use as a solvent for cellulose acetate and dyes, in inks and resins, as a perfume fixative, as a bactericidal agent, in organic synthesis of plasticizers, germicides and pharmaceuticals, and in insect repellents may result in its release to the environment through various waste streams. If released to air, a vapor pressure of 0.007 mm Hg at 25 deg C indicates 2-phenoxyethanol will exist solely as a vapor in the atmosphere. Vapor-phase 2-phenoxyethanol will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 11.8 hours. Monitoring data have shown that 2-phenoxyethanol can be removed from the atmosphere via precipitation including snow. 2-Phenoxyethanol does not absorb at wavelengths >290 nm, and therefore is not expected to be susceptible to direct photolysis by sunlight. If released to soil, 2-phenoxyethanol is expected to have very high mobility based upon an estimated Koc of 15. Volatilization from moist soil surfaces is not expected to be an important fate process based upon an estimated Henry's Law constant of 4.9X10-8 atm-cu m/mole. If released into water, 2-phenoxyethanol is not expected to adsorb to suspended solids and sediment based upon the estimated Koc. Volatilization from water surfaces is not expected to be an important fate process based upon this compound's estimated Henry's Law constant. Theoretical BODs of 2% (5-day), 71% (10-day), 50% (20-day) and 80% (20-day) have been reported for 2-phenoxyethanol indicating it will be largely removed during biological waste treatment. 2-Phenoxyethanol is not expected to undergo hydrolysis in the environment due to the lack of functional groups that hydrolyze under environmental conditions. Occupational exposure to the 2-phenoxyethanol occurs through inhalation of vapor and dermal contact. Its use as solvent for inks, resins and cellulose acetate and its use as a perfume fixative could expose the general population through dermal contact and inhalation of vapor. (SRC)
Probable Routes of Human Exposure:
According to the 2006 TSCA Inventory Update Reporting data, the number of persons reasonably likely to be exposed in the industrial manufacturing, processing, and use of 2-phenoxyethanol is 1000 or greater; the data may be greatly underestimated(1).
NIOSH (NOES Survey 1981-1983) has statistically estimated that 111,040 workers (46,637 of these were female) were potentially exposed to 2-phenoxyethanol in the US(1). Occupational exposure to the 2-phenoxyethanol occurs through inhalation of vapor and dermal contact. Its use as solvent for inks, resins and cellulose acetate and its use as a perfume fixative could expose the general population through dermal contact and inhalation of vapor(SRC).
Artificial Pollution Sources:
2-Phenoxyethanol's production and use as a solvent for cellulose acetate and dyes, in inks and resins, as a perfume fixative, as a bactericidal agent, in organic synthesis of plasticizers, germicides and pharmaceuticals and in insect repellents(1) may result in its release to the environment through various waste streams(SRC).
Environmental Fate:
TERRESTRIAL FATE: Based on a classification scheme(1), an estimated Koc value of 15(SRC), determined from a structure estimation method(2), indicates that 2-phenoxyethanol is expected to have very high mobility in soil(SRC). Volatilization of 2-phenoxyethanol from moist soil surfaces is not expected to be an important fate process(SRC) given an estimated Henry's Law constant of 4.9X10-8 atm-cu m/mole(SRC), based upon its vapor pressure, 0.007 mm Hg at 25 deg C(3), and water solubility, 2.6X10+4 mg/L(4). One biodegradation study reported theoretical 2-phenoxyethanol BODs of 2% (5-day), 71% (10-day), and 80% (20-day)(3); a theoretical 20-day BOD of 50%(3) indicates biodegrdaation may be an important environmnetal fate process in soil(SRC).
AQUATIC FATE: Based on a classification scheme(1), an estimated Koc value of 15(SRC), determined from a structure estimation method(2), indicates that 2-phenoxyethanol is not expected to adsorb to suspended solids and sediment(SRC). Volatilization from water surfaces is not expected(3) based upon an estimated Henry's Law constant of 4.9X10-8 atm-cu m/mole(SRC), derived from its vapor pressure, 0.007 mm Hg at 25 deg C(4), and water solubility, 2.6X10+4 mg/L(5). According to a classification scheme(6), an estimated BCF of 1.5(SRC), from its log Kow of 1.16(7) and a regression-derived equation(8), suggests the potential for bioconcentration in aquatic organisms is low. One biodegradation study reported theoretical 2-phenoxyethanol BODs of 2% (5-day), 71% (10-day), and 80% (20-day)(4); a theoretical 20-day BOD of 50% indicates a compound will largely be removed during biological waste treatment(4). 2-Phenoxyethanol is not expected to undergo hydrolysis in the environment due to the lack of functional groups that hydrolyze under environmental conditions(3).
ATMOSPHERIC FATE: According to a model of gas/particle partitioning of semivolatile organic compounds in the atmosphere(1), 2-phenoxyethanol, which has a estimated vapor pressure of 0.007 mm Hg at 25 deg C(2), is expected to exist solely as a vapor in the ambient atmosphere. Vapor-phase 2-phenoxyethanol is degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals(SRC); the half-life for this reaction in air is estimated to be about 11.8 hours(SRC), calculated from its rate constant of 3.27X10-11 cu cm/molecule-sec at 25 deg C(SRC) that was derived using a structure estimation method(3). Monitoring data have shown that 2-phenoxyethanol can be removed from the atmosphere via precipitation such as snow(4). 2-Phenoxyethanol does not absorb at wavelengths >290 nm(5), and therefore is not expected to be susceptible to direct photolysis by sunlight(SRC).
Environmental Biodegradation:
AEROBIC: For 2-phenoxyethanol, theoretical BODs of 2% (5-day), 71% (10-day), and 80% (20-day) have been measured(1); a theoretical 20-day BOD of 50% indicates a compound will largely be removed during biological waste treatment(1).
Environmental Abiotic Degradation:
The rate constant for the vapor-phase reaction of 2-phenoxyethanol with photochemically-produced hydroxyl radicals has been estimated as 3.27X10-11 cu cm/molecule-sec at 25 deg C(SRC) using a structure estimation method(1). This corresponds to an atmospheric half-life of about 11.8 hours at an atmospheric concentration of 5X10+5 hydroxyl radicals per cu cm(1). The UV spectrum for an aqueous solution of 2-phenoxyethanol does not show any absorbance above 290 nm(2) which indicates that 2-phenoxyethanol will not directly photolyze in the environment(SRC). 2-Phenoxyethanol is not expected to undergo hydrolysis in the environment due to the lack of functional groups that hydrolyze under environmental conditions(3).
Environmental Bioconcentration:
An estimated BCF of 1.5 was calculated in fish for 2-phenoxyethanol(SRC), using a log Kow of 1.16(1) and a regression-derived equation(2). According to a classification scheme(3), this BCF suggests the potential for bioconcentration in aquatic organisms is low(SRC).
Soil Adsorption/Mobility:
Using a structure estimation method based on molecular connectivity indices(1), the Koc of 2-phenoxyethanol can be estimated to be 15(SRC). According to a classification scheme(2), this estimated Koc value suggests that 2-phenoxyethanol is expected to have very high mobility in soil.
Volatilization from Water/Soil:
The Henry's Law constant for 2-phenoxyethanol is estimated as 4.9X10-8 atm-cu m/mole(SRC) derived from its vapor pressure, 0.007 mm Hg at 25 deg C(1), and water solubility, 2.6X10+4 mg/L(2). This Henry's Law constant indicates that 2-phenoxyethanol is essentially nonvolatile from water surfaces(3). 2-Phenoxyethanol's Henry's Law constant indicates that volatilization from moist soil surfaces is not expected to occur(SRC). 2-Phenoxyethanol is not expected to volatilize from dry soil surfaces(SRC) based upon the vapor pressure of 0.007 mm Hg at 25 deg C(3).
Environmental Water Concentrations:
DRINKING WATER: 2-Phenoxyethanol was qualitatively detected in drinking water concentrates collected in Cincinnati, OH on Oct 17, 1978(1).
GROUND WATER: 2-Phenoxyethanol concentrations of less than 5 ppm were detected in well waters collected in the vicinity of two industrial factories in Spain in 1984(1).
Effluent Concentrations:
2-Phenoxyethanol has been detected in wastewater effluents from the following industries: paint and ink, organics & plastics, photographic, and mechanical products(1). 2-Phenoxyethanol was detected in samples collected from oil reclaiming wastewaters(2). 2-Phenoxyethanol was qualitatively detected in wastewater samples collected from the Iona Island municipal treatment facility (British Columbia) in 1983(3).
Atmospheric Concentrations:
RAIN/SNOW: Snow samples collected in early March (year not specified) at Lapland, Finland and Moscow, Russia contained 2-phenoxyethanol concentrations of 0.28 and 0.04 ug/kg respectively(1).
Environmental Standards & Regulations:
TSCA Requirements:
Pursuant to section 8(d) of TSCA, EPA promulgated a model Health and Safety Data Reporting Rule. The section 8(d) model rule requires manufacturers, importers, and processors of listed chemical substances and mixtures to submit to EPA copies and lists of unpublished health and safety studies. 2-Phenoxyethanol is included on this list.
Atmospheric Standards:
This action promulgates standards of performance for equipment leaks of Volatile Organic Compounds (VOC) in the Synthetic Organic Chemical Manufacturing Industry (SOCMI). The intended effect of these standards is to require all newly constructed, modified, and reconstructed SOCMI process units to use the best demonstrated system of continuous emission reduction for equipment leaks of VOC, considering costs, non air quality health and environmental impact and energy requirements. Ethylene glycol monophenyl ether is produced, as an intermediate or final product, by process units covered under this subpart.
FDA Requirements:
Ethylene glycol monophenyl ether is an indirect food additive for use only as a component of adhesives.
Chemical/Physical Properties:
Molecular Formula:
C8-H10-O2
Molecular Weight:
138.16
Color/Form:
Oily liquid
Colorless liquid
Odor:
Faint aromatic odor
Taste:
Burning taste
Boiling Point:
245.2 deg C
Melting Point:
14 deg C
Density/Specific Gravity:
1.1094 at 20 deg C/20 deg C
Dissociation Constants:
pKa = 15.10 at 25 deg C
Heat of Combustion:
958 kcal/mole
Octanol/Water Partition Coefficient:
log Kow = 1.16
Solubilities:
Freely soluble in alcohol, ether, and sodium hydroxide
Soluble in ethanol, alkali, chloroform
2.67 g/100 mL water
In water, 2.6X10+4 mg/L at 20 deg C
Spectral Properties:
Index of refraction: 1.534 at 20 deg C/D
SADTLER REF NUMBER: 248 (IR, PRISM); 84 (IR, GRATING); 99 (UV); 506 (NMR, VARIAN)
MASS: 21124 (NIST/EPA/MSDC Mass Spectral Database, 1990 version); 1240 (Atlas of Mass Spectral Data, John Wiley & Sons, New York)
IR: 1055 (Coblentz Society spectral collection)
UV: 99 (Sadtler Research Laboratories spectral collection)
Raman: 201 (Sadtler Research Laboratories spectral collection)
1H NMH: 506 (Varian Associates NMR spectra collection)
Surface Tension:
42.0 dynes/cm
Vapor Density:
4.8 (Air = 1)
Vapor Pressure:
0.007 mm Hg at 25 deg C
Relative Evaporation Rate:
< 0.01 (Butyl acetate = 1.0)
Viscosity:
20.5 centistokes at 25 deg C
Other Chemical/Physical Properties:
Bulk density: 9.2 lb/gal
% in saturated air: 0.00096 at 25 deg C
Liquid; bp: 243 deg C /2-Phenoxyethanol acetate/
Henry's Law constant = 4.9X10-8 atm-cu m/mole at 25 deg C (est)
Hydroxyl radical reaction rate constant = 3.27X10-11 cu cm/molecule-sec at 25 deg C (est)
Chemical Safety & Handling:
Hazards Summary:
The major hazards encountered in the use and handling of 2-phenoxyethanol stem from its toxicologic properties. Toxic by all routes (inhalation, ingestion, and dermal contact), exposure to this faintly aromatic, colorless, oily liquid may occur from its use as a fixative for cosmetics, perfumes, and soaps; as a bactericide and insect repellant; as a solvent for cellulose acetate,dyes, stamp pad, ball point, and specialty inks; as a chemical intermediate for carboxylic acid esters (eg, acrylate, maleate) and polymers (eg, formaldehyde, melamine); and as a preservative for human specimen used for dissection and demonstrations in anatomical studies. Effects from exposure may include eye irritation, headache, tremors, and CNS depression. If contact should occur, irrigate exposed eyes with copious amounts of tepid water for at least 15 minutes, and wash exposed skin thoroughly with soap and water. 2-Phenoxyethanol must be preheated before ignition can occur. If this substance is involved in a fire, water spray gently applied to the surface will cause a frothing which will extinguish the fire.
Skin, Eye and Respiratory Irritations:
A skin and severe eye irritant.
Fire Potential:
Combustible when exposed to heat or flame ... .
NFPA Hazard Classification:
Health: 3. 3= Materials that, on short exposure, could cause serious temporary or residual injury, including those requiring protection from all bodily contact. Fire fighters may enter the area only if they are protected from all contact with the material. Full protective clothing, including self-contained breathing apparatus, coat, pants, gloves, boots, and bands around legs, arms, and waist, should be provided. No skin surface should be exposed.
Flammability: 1. 1= This degree includes materials that must be preheated before ignition will occur, such as Class IIIB combustible liquids and solids and semi-solids whose flash point exceeds 200 deg F (93.4 deg C), as well as most ordinary combustible materials. Water may cause frothing if it sinks below the surface of the burning liquid and turns to steam. However, a water fog that is gently applied to the surface of the liquid will cause frothing that will extinguish the fire.
Instability: 0. 0= This degree includes materials that are normally stable, even under fire exposure conditions, and that do not react with water. Normal fire fighting procedures may be used.
Flash Point:
260 deg F (127 deg C) (Closed cup)
Fire Fighting Procedures:
To fight fire, use CO2, dry chemical.
Hazardous Reactivities & Incompatibilities:
Can react vigorously with oxidizing materials.
Hazardous Decomposition:
When heated to decomposition it emits acrid smoke and irritating fumes.
Preventive Measures:
SRP: The scientific literature for the use of contact lenses by industrial workers is inconsistent. The benefits or detrimental effects of wearing contact lenses depend not only upon the substance, but also on factors including the form of the substance, characteristics and duration of the exposure, the uses of other eye protection equipment, and the hygiene of the lenses. However, there may be individual substances whose irritating or corrosive properties are such that the wearing of contact lenses would be harmful to the eye. In those specific cases, contact lenses should not be worn. In any event, the usual eye protection equipment should be worn even when contact lenses are in place.
REASONABLE HANDLING PRECAUTIONS, PLUS PARTICULAR CARE TO PREVENT CONTACT WITH THE EYES, SHOULD PREVENT ANY SERIOUS TOXIC EFFECTS.
Stability/Shelf Life:
STABLE IN PRESENCE OF ACIDS & ALKALIES.
Disposal Methods:
SRP: The most favorable course of action is to use an alternative chemical product with less inherent propensity for occupational harm/injury/toxicity or environmental contamination. Recycle any unused portion of the material for its approved use or return it to the manufacturer or supplier. Ultimate disposal of the chemical must consider: the material's impact on air quality; potential migration in soil or water; effects on animal and plant life; and conformance with environmental and public health regulations.
Occupational Exposure Standards:
Manufacturing/Use Information:
Uses:
Fixative for perfumes, in organic synthesis; as a bactericide in conjunction with quaternary ammonium compounds
Solvent for cellulose acetate, dyes; inks, resins; perfume fixative; bactericidal agent; organic synthesis of plasticizers, germicides, pharmaceuticals; insect repellents
MEDICATION (See also: Therapeutic Uses)
Manufacturers:
Dow Chemical USA, Hq, 2030 Dow Center, Midland, MI 48642, (989) 636-1000; Production site: Freeport, TX 77541
Eastman Chemical Company, P.O. Box 431, Kingsport, TX 37662, (423) 229-2000, Chemicals and Fibers Group; Production site: Longview, TX 75607
Methods of Manufacturing:
A mixture of ethylene chlorohydrin in 30% aqueous NaOH may be added to phenol at 100-110 deg C to give 2-phenoxyethanol in 98% yield.
Obtained by treating phenol with ethylene oxide in an alkaline medium.
Produced by the hydroxyethylation of phenol ... in the presence of alkali-metal hydroxides or alkali metal borohydrides.
Formulations/Preparations:
Grade: Technical
U. S. Production:
(1979) PROBABLY GREATER THAN 4.54X10+6 GRAMS
(1981) PROBABLY GREATER THAN 4.54X10+6 GRAMS
Ethanol, 2-phenoxy- is listed as a High Production Volume (HPV) chemical (65FR81686). Chemicals listed as HPV were produced in or imported into the U.S. in >1 million pounds in 1990 and/or 1994. The HPV list is based on the 1990 Inventory Update Rule. (IUR) (40 CFR part 710 subpart B; 51FR21438).

